Navigating a Complex Maze
By Mike King

Illustration by Mark Allen Miller
 |
Isolated outside its habitat and under the refractive prowess of an electron microscope, HIV has earned grudging respect from the scientists who study it.
HIV consists of just two strands of genetic material together with a small number of enzymes wrapped inside a viral envelope. It measures just 1/10,000th of a millimeter in diameter. The glycoprotein spikes on its outer envelope give the impression that the virus is reaching out to glom on to anything in its path, which is exactly what it is programmed to do. For a retrovirus like HIV to survive, it must get inside host cells and use the machinery of those cells to reproduce.
If nothing else, after three decades of studying HIV, we know that it can perform that task with remarkable efficiency—fusing to lymphocytes and other protective cells in the body, injecting its viral core into them, and assembling thousands of new viral cores that deploy and destroy the human immune system. If HIV is left untreated, infections—even the most common kind—run rampant, wasting, then killing, the body.
Scientists at Emory and elsewhere have designed drugs to help people with HIV live longer. But the quest to eradicate the virus—to come up with a vaccine that renders it harmless—remains a major, and often frustrating, scientific challenge.
Despite a wealth of information about HIV, we still don't know how to develop an effective protective vaccine against it, in part because the outer envelope is able to mutate in ways that are different in each infected person.
"We have made great strides in public health and prevention, in drugs and treatment protocols as well as in vaccine development—all of these being areas where Emory has made major contributions—but we may be at the point where we need to know what we don't know about HIV," says Emory's Eric Hunter.
 |
Exploring new avenues
A critical mass of investigators at Emory is asking those questions to pinpoint the right direction forward. "It's pretty amazing to sit back and watch how it has all come together at Emory," says James Bradac, chief of preclinical research and development of the vaccine research program of the National Institute of Allergy and Infectious Diseases, the arm of the NIH responsible for most research involving HIV/AIDS. "I use the analogy of a baseball franchise. Upper management made a commitment to create a world-class vaccine center and build a solid team of great investigators. And with that has come grant money to make it happen."
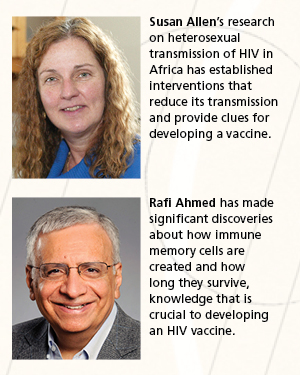
In July the NIH announced a $7 million award to three Emory investigators—Rafi Ahmed, Bali Pulendran, and Guido Silvestri—to support multidisciplinary research into triggering immune responses that could prevent or contain HIV from replicating itself in host cells. The research, funded as part of the Center for HIV/AIDS Vaccine Immunology, cuts across the work of these investigators and is illustrative of the research avenues that Emory scientists are exploring.
Just last year a group of Emory researchers received a grant—$6 million each year for at least five years—to explore ways to make a better HIV vaccine using the simian immunodeficiency virus (SIV) as a model in nonhuman primates. SIV has long intrigued scientists because some primates infected with the virus never progress to the disease stage. With these grants, Emory Vaccine Center scientists are working together to understand how to induce neutralizing antibody responses in virus-fighting cells. For example, they are experimenting with a powerful nanoparticle vaccine pioneered in Pulendran's lab (nanoparticles are tiny, super-reactive particles that can deliver an immune-triggering response directly within host cells) and novel cytokine adjuvants developed in Rama Amara's lab (cytokines are small cell-signaling protein molecules used in intercellular communication).
 |
The goal of this work is to find new approaches to augmenting immunity to SIV infection across mucosal barriers. Infection through the mucosal tissue during sex is the primary mechanism for spreading HIV in humans.
Obstacles in the path
The naïve 1980s optimism about developing a vaccine has long since faded. Despite progress, the AIDS epidemic is far from being crushed, especially in the developing world.
In wealthy nations, where access to the drugs needed to treat it is usually not a problem, HIV no longer is seen as the threat it once was. In 1995, more than 50,000 Americans a year were dying of AIDS. The yearly death toll now is below 20,000, about the same number of people who die of pancreatic cancer. Nevertheless, nearly 60,000 individuals continue to be infected with HIV in the United States each year.
In developing countries, especially in sub-Saharan Africa, the toll remains dire. In 2010 an estimated 34 million people worldwide were living with HIV/AIDS, about 10% of them children. That same year nearly 2 million people died of the disease. HIV leaves millions of children orphaned and destroys whole communities in poor countries, where it continues to be spread primarily through heterosexual contact.
 |
Even with life-prolonging drugs against HIV, Hunter says, he doesn't see "any way to treat ourselves out of this disease." And because public health measures to prevent the disease in developing countries remain woefully underfunded, the demand for a vaccine is greater now than ever.
From the initial hope of developing antiviral medicines like those used against other sexually transmitted diseases to vaccines targeting genes and specific cellular responses, the focus of vaccine research has changed several times, Hunter says. So far, the vaccines that have made their way into human trials have been only modestly successful.
Following the road not taken
More recently, vaccine researchers have re-focused on the earliest events in the HIV infection cycle, specifically on how the immune system responds to challenges the virus presents. There is some hope, Hunter says, that combinations of agents—and new ways of delivering them (such as Pulendran's nanoparticle experiments)—will confer a higher degree of protection against the ability of HIV to infect cells and mutate before the body's immune system can arm against it.
For Cynthia Derdeyn, the new focus takes her back to days as a postdoctoral fellow in Hunter's laboratory at the University of Alabama at Birmingham (UAB) prior to joining Emory in 2004. It was there that investigators began to realize that the virus that gets transmitted from one person to another may have "special properties" that could be exploited in vaccine development, she says.
"Eight years later, we still don't know what those special properties are," Derdeyn says. "And we still don't have a sufficient understanding of how HIV is transmitted sexually." She believes the answer lies in part in ongoing research about how the virus is transmitted within heterosexual couples in Africa.
Transmission among homosexual partners was an early focus of HIV research, leading to important discoveries about the virus. More recently, Derdeyn and Hunter turned their attention to heterosexual transmission in Africa, where distinct variations of the virus are found.
A big part of that research relies on a large repository of health information and reagents compiled by Susan Allen, another UAB-to-Emory transplant. Derdeyn and Hunter used samples from Allen's work in Rwanda and Zambia with "discordant couples"—male and female sex partners, one of whom is HIV positive and the other isn't—to show that HIV most of the time runs into a genetic bottleneck during heterosexual transmission.
 |
Using the DNA sequences for the gene that encodes a glycoprotein on the outer coat of HIV, the Emory researchers discovered that a single viral variant in each donor established infection in the recipient partner. These transmitted variants are all genetically distinct, but they share properties in their glycoproteins that may make them more vulnerable to a vaccine. These findings suggest that infection may be triggered by specific viral variants more so than by chance.
By understanding these distinct features of the virus, scientists may eventually be able to target them with microbicides or adapt a vaccine that proves much more effective against heterosexual transmission.
"Back in the early 2000s, only a few investigators were really looking at the African viruses," Derdeyn says. "They were mainly using the data to study transmission linkage between partners from a public health perspective. We decided this was a good opportunity to take a deeper look into what the transmitted virus looks like."
Thus is the nature of discovery.
At the dawn of the fourth decade of the AIDS epidemic as we know it, perhaps the biggest obstacle in eradicating it lies in acknowledging that we still have a lot to learn. EH
Related Video
"James Curran reflects on 31 years of AIDS"
Related Story in This Issue
Related Links
"Certain mutations give HIV infection an advantage that sticks"
"Emory to receive $6 million for AIDS vaccine research"
"NIH awards $9 million to Emory CFAR"
More HIV/AIDS Stories at Emory University
HIV/AIDS Media Kit - Emory Health Sciences






