Ready Phase One
Phase I clinical trials are the standing-on-the-high-dive moments of cancer research
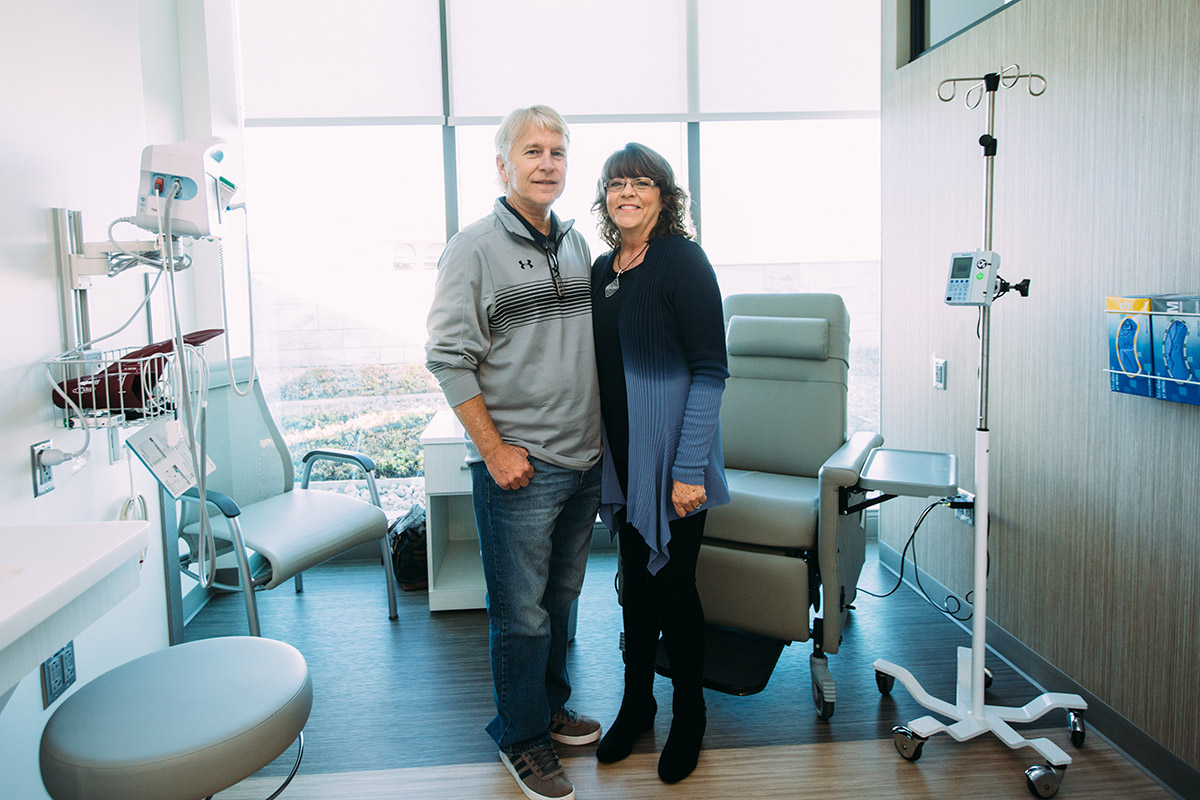
"I knew what was going to happen, I knew I was going to die,” says Michael Golden.
In 2010, Golden (above, left) was diagnosed with advanced cancer and was advised to go home and put his affairs in order. Renal cell cancer had invaded one of Golden’s kidneys and extended into the vena cava—the largest vein in the body—all the way to his heart.
Golden’s oncologist kept looking for other avenues of hope and found Winship’s urologic cancer surgeon, Viraj Master. The dire outlook had discouraged Golden, and he would not take Master’s call.
“Finally, my oncologist from Kaiser called the house and said, there’s a doctor from Emory calling you, you need to talk to him. So Dr. Master called back, and I answered his call. We talked and he told me what he thought we could do,” Golden remembers.
“I never will forget it. It was a rush of emotions like you’ve never felt in your life. From going from knowing that you have just a few months to live, to, you know, he mentioned the word ‘cure.’ ”
Master specializes in exactly the type of surgery Golden needed. He enlisted a vascular surgeon and cardiac surgeon to work alongside him, and together they performed a nearly 15-hour surgery to remove a kidney and the tumor that snaked up the length of Golden’s vena cava.
The surgery kept him alive. Years of battling renal cancer with different chemotherapy drugs followed, but the cancer kept coming back. In 2017, Golden started a new Phase I clinical trial testing a combination of an approved small molecule anticancer drug with an investigational drug targeting tumor-based metabolic pathways. The new therapy worked, but it also threatened to shut down his remaining kidney and his life. His medical oncology doctor, Winship’s M. Asim Bilen, decided to keep him on the investigational drug but eliminate the other drug, which was causing the side effects. He continued on the investigational drug.
“I feel amazing. I’ve gained back 35 pounds, my hair has grown back, I haven’t felt this good in literally over 10 years,” Golden says today.
And the 61-year-old retired Atlantan looks as healthy as he feels. Which is amazing, considering that survival rates for advanced kidney cancer have not been good.
“They tell you up front that you’re not taking this drug as a cure, you’re taking it to help other folks, and that was fine with me. We were excited, we weren’t scared, we were looking forward to getting something that might work,” says Golden.
Before an experimental anticancer drug is approved by the US Food and Drug Administration for general public usage, it goes through three phases of testing in humans. All three phases are crucial to establish the safety and efficacy of new therapies.
But Phase I clinical trials, the first time a new treatment is tested in humans, are the standing-on-the-high-dive moments of cancer research.
Sometimes patients go into Phase I trials because there aren’t any other good options left. Often, they’ve spent years on standard-of-care treatments that have had some success but are no longer working.
Phase I trials are not always a last-ditch option. Sometimes doctors recommend a Phase I trial to a patient who’s at an earlier point in treatment because the new therapy has the potential to be more effective than other available treatments for that patient’s disease.
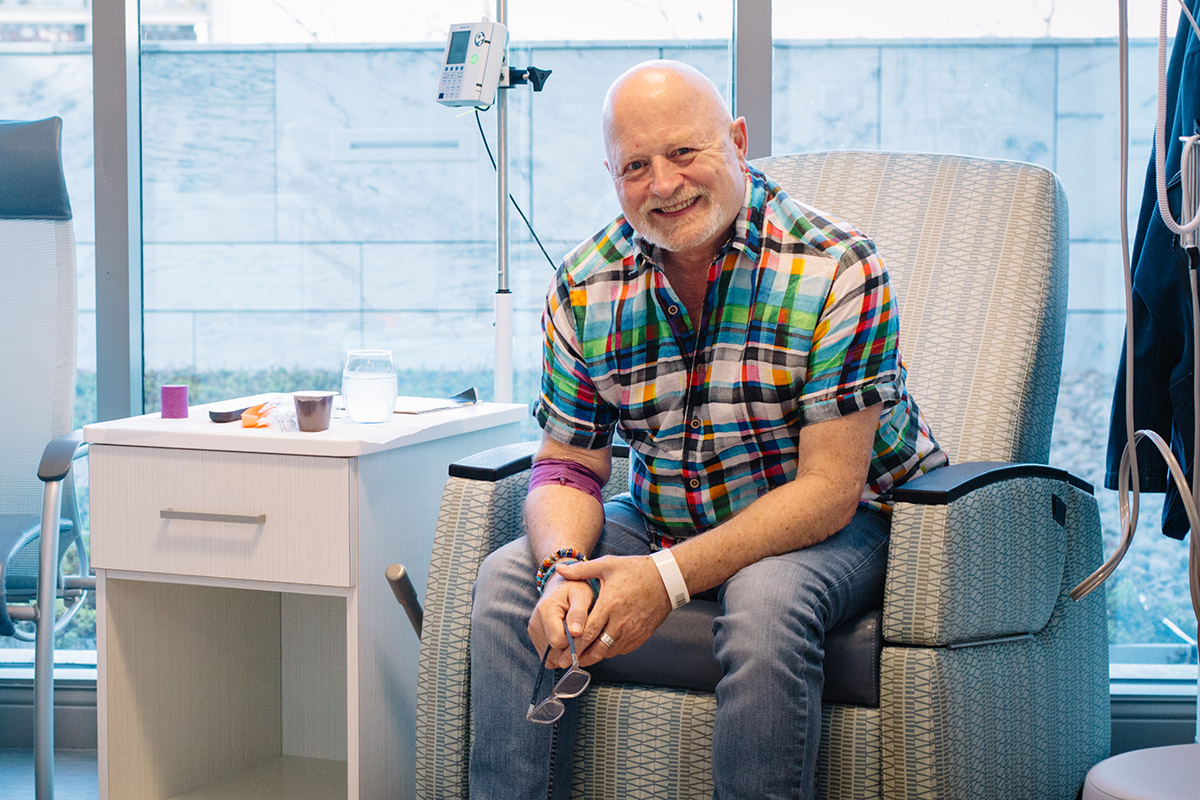
Tom Broyles, an artist from Decatur with metastatic renal cell cancer, went into a Phase I trial combining immunotherapy with a new drug. He says the treatment started working right away. The new Phase I Unit, he says, keeps in mind “quality of life” as well as quality of care.
Tom Broyles, a semiretired artist from Decatur, is another patient with renal cell cancer. He started out with a less dire prognosis but after a kidney was removed, the cancer came back and metastasized. Having only one kidney complicated his ability to have radiation and chemotherapy, so in 2017 Broyles went into a Phase I trial combining immunotherapy with a new drug. He says the treatment started working right away.
“They were like whoa—the cancer has reduced more than they ever expected. Lo and behold by the end of the summer, there was no detection. This all happened within one year,” says Broyles, who now spends his time in his art studio, having retired from a position as a financial systems analyst with the city of Atlanta.
Everyone wishes for these kind of results, but the success rate of new cancer drugs has been historically low.
Patients are carefully advised about the downsides of a Phase I trial—the possible risks and side effects, the time commitment and extra tests required, and perhaps most important, that the treatment may not benefit the patient.
The consent agreement spells it out: “Your condition may improve while you are in this study, but it may not, and it may even get worse.”
Instead, patients are told that the greatest value of being in a Phase I trial is to the patients who come after them; hopefully the study will either bring a new treatment to light, or rule out an ineffective one.
But Phase I trials require a significant infrastructure and commitment of resources: specially trained nurses, pharmacists, support staff, a knowledgeable research staff to handle massive amounts of regulatory paperwork, special equipment, and a dedicated space. So when Winship Cancer Institute of Emory University decided to expand and upgrade its original Phase I Unit, it pulled out all the stops.
“The investment of patients, physicians, nurses, other clinicians, health system leaders, and research staff in the design and execution of this unit has been nothing short of amazing,” says R. Donald Harvey, who has been the director of the Phase I Clinical Trials section since Winship launched the first unit in 2009. “We will continue to grow the Phase I program and benefit an even greater population in the years to come.”

Making staff and patients partners in the design process was essential to the new Phase I Unit. Melissa Childress, vice president of Winship Cancer Services, says Winship is taking this approach in construction and remodeling projects throughout the Emory Healthcare system.
Harvey supported the Phase I lead nurse practitioner, Colleen Lewis, and her team to take the lead in designing the new unit. They started with a big unfinished space on the fourth floor of the new Emory University Hospital Tower, triple the size of the original unit, and assembled a multidisciplinary team of patients, nurses, physicians, and research staff.
The team spent weeks together sketching out floor plans and drawing up lists of “hopes” and “please considers,” then saw their ideas transformed into a life-size 3-D model of cardboard where they ran clinical scenarios and tweaked the design.
Because the tolerance for a new treatment is unknown, medical staff document every nuance of the
patient experience and must be ready to respond to unexpected reactions. The challenge was to create a space focused on patient safety and comfort, yet able to support the painstaking demands of rigorous clinical research.
Construction started in summer 2018 and finished by the end of the year. The new unit enables staff to treat more patients with the highest standards of safety and efficiency, and it also prioritizes the comfort of patients and caregivers with specially designed treatment areas, furniture (like heated treatment chairs), and amenities. The treatment bays all have full-length windows that provide light as well as campus and skyline views.
“The focus on the patient is clear from the moment you walk in,” says Lewis.
The physical surroundings reflect the same concern for a patient’s quality of life as the care. “All those things we do day to day, they don’t forget about it: We want to treat you but we want your quality of life to be as positive and as energetic as it can be,” says Broyles.
Phase I clinical trials go to the heart and soul of a cancer institute’s mission to turn scientific discoveries into therapies that help cancer patients.
The Phase I Unit started treating patients in January 2019. Golden and Broyles were among the first patients in the door. In its first year of operation, the Winship Phase I Clinical Trials Unit received the highest possible patient satisfaction ratings.
“Phase I clinical trials are where we find out if scientific discoveries have the potential to become new cancer treatments, and there’s no other place in Georgia where you can get therapies like the kind you get in this dedicated Phase I Unit,” says Walter Curran Jr., executive director of Winship Cancer Institute.
You might think that being one of the first people to try a new cancer drug would be anxiety provoking.
But patients like Golden, Broyles, and others say that taking part in this early phase of cancer research makes them feel a part of the discovery process and gives them hope that even if their trial doesn’t work, there will be others that may.




